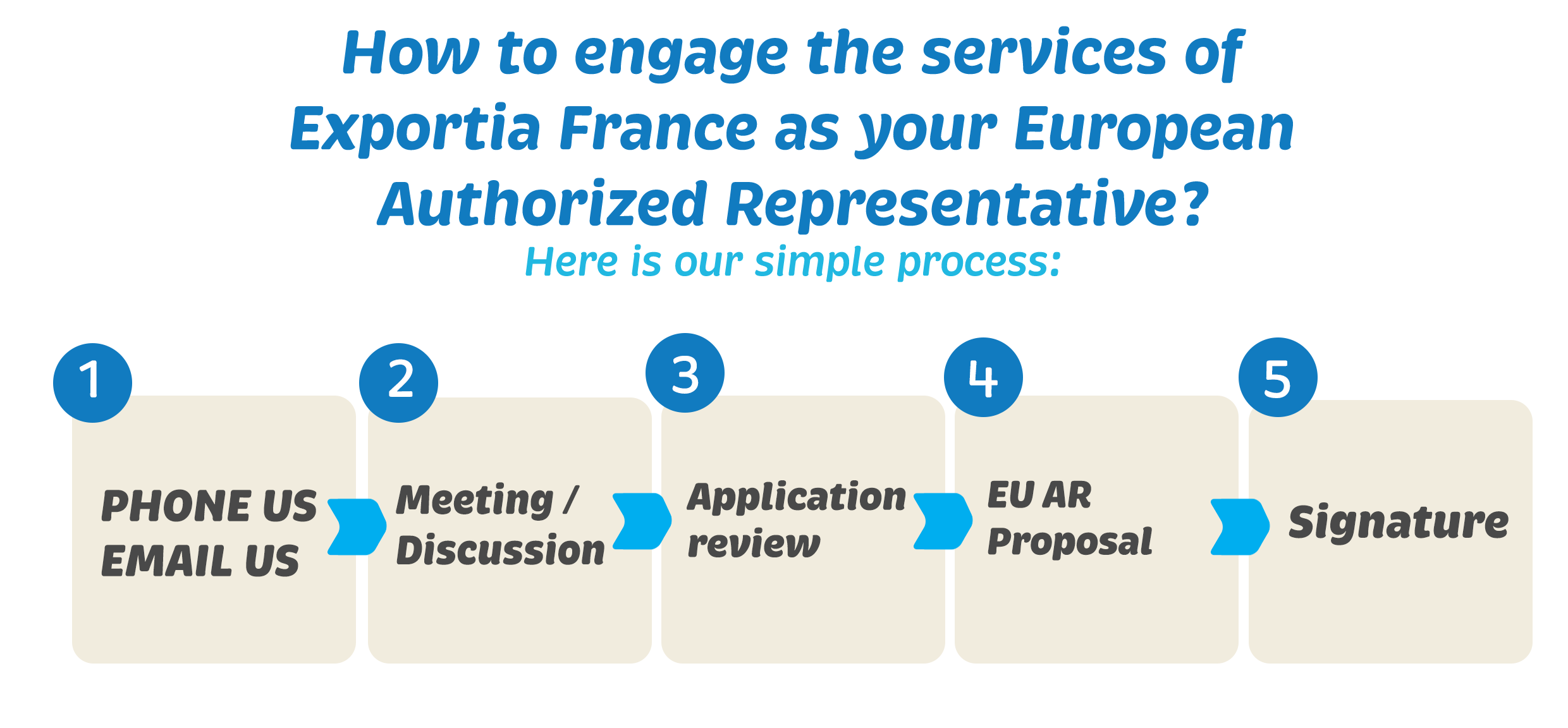
New regulatory requirements under the Medical Device Regulation (MDR)
Under the European Directive for Medical Devices (EU 2017/745), a manufacturer of a device, which is not established in a member State of the European Union, has to appoint an authorized representative in the Union in order to comply with the directive.
This European Authorized Representative represents your business for European Union Regulatory Authorities and the local competent authority. This role is defined in article 11 of the European 2017/745.
Here is an extract of the Article 11 of the regulation. Here is a summary of the tasks that Exportia France will conduct:
(a) verify that the EU declaration of conformity and technical documentation have been drawn up and, where applicable, that an appropriate conformity assessment procedure has been carried out by the manufacturer;
(b) keep available a copy of the technical documentation, the EU declaration of conformity and, if applicable, a copy of the relevant certificate, including any amendments and supplements at the disposal of competent authorities for a period of 10 or 15 years.
(c) comply with the registration obligations laid down in Article 31 and verify that the manufacturer has complied with the registration obligations laid down in Articles 27 and 29;
(d) in response to a request from a competent authority, provide that competent authority with all the information and documentation necessary to demonstrate the conformity of a device, in an official Union language determined by the Member State concerned;
(e) forward to the manufacturer any request by a competent authority of the Member State in which the authorised representative has its registered place of business for samples, or access to a device and verify that the competent authority receives the samples or is given access to the device;
(f) cooperate with the competent authorities on any preventive or corrective action taken to eliminate or, if that is not possible, mitigate the risks posed by devices;
(g) immediately inform the manufacturer about complaints and reports from healthcare professionals, patients and users about suspected incidents related to a device for which they have been designated;
(h) terminate the mandate if the manufacturer acts contrary to its obligations under this Regulation.
Source: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0745
EU Authorized Representative under the MDR
Exportia France SAS, based in Paris and its legal representative Christelle Damiens offers an independent European Authorized Representative service for non-Europe based medical device companies.

In France
At the heart of a major European Medical Device market, France, Exportia is well positioned
- to respond to the local authority requests in an efficient manner
- to communicate, if required, with European distributors and customers. PRRC is a French native speaker, and fluent in German and English.

An Independent Representative
Of course, you can choose to give this mandate to your distributor or your importer, but some of the advantages of having in an independent EU-Authorized Representative are:
- our focus will be 100% oriented on the manufacturer’s needs and the competent authorities requests, without any commercial interests interfering
- it gives the freedom to the manufacturer to shift and change its distribution strategy while leaving the role and responsibilities of the European Authorized Representative undisturbed.

Large range of Medical Device experience in the EU
At Exportia, we have broad experience in the medical device sector in Europe accumulated over the last 16 years. Our Person Responsible for Regulatory Compliance(PRRC), Christelle has been involved with a very diverse range of products (class I, II and II) ranging from eHealth software, implants, x-ray, dermal substitutes, patient risk management solutions to ultrasonic irrigators cleaners just to name a few.